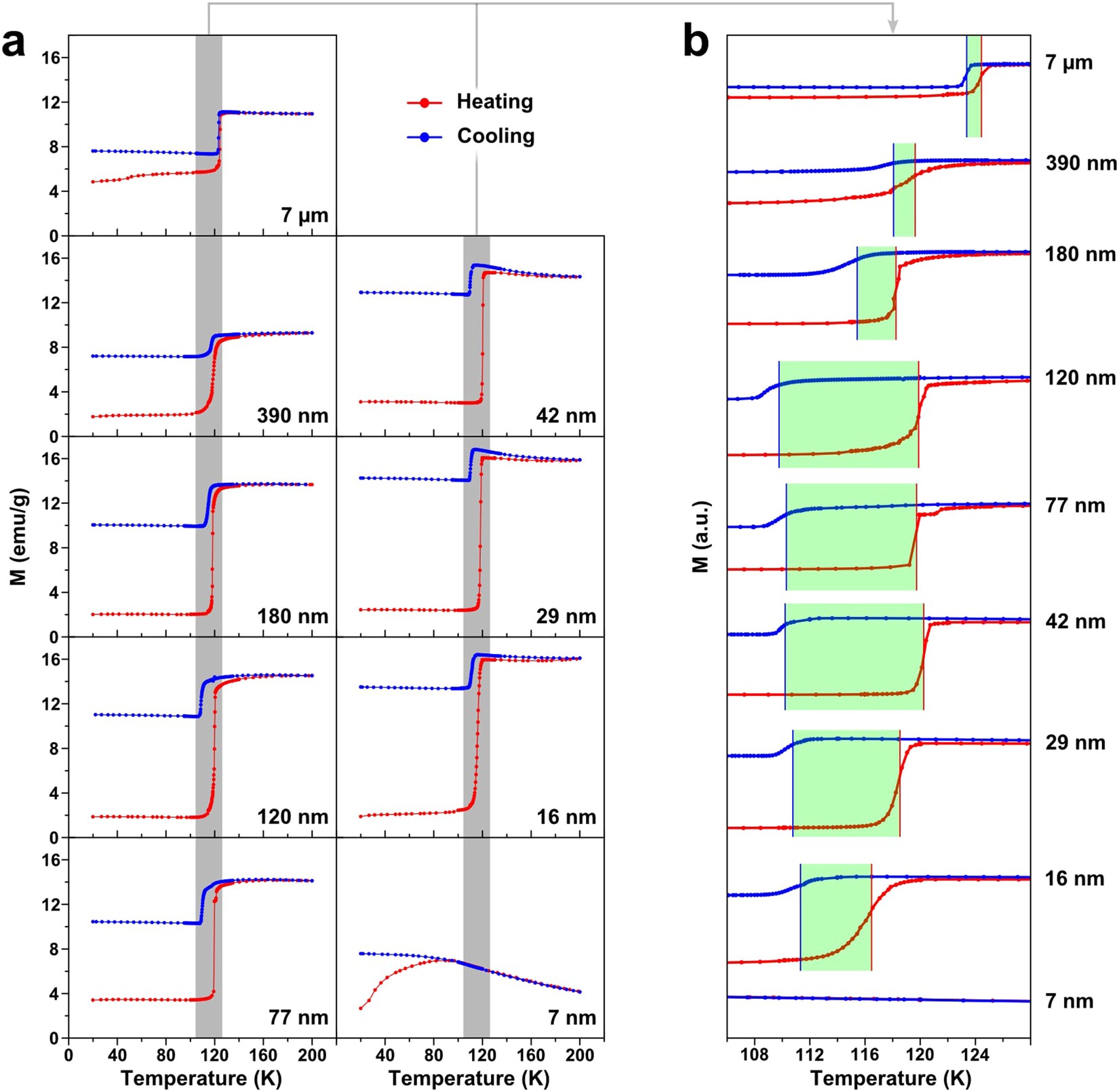
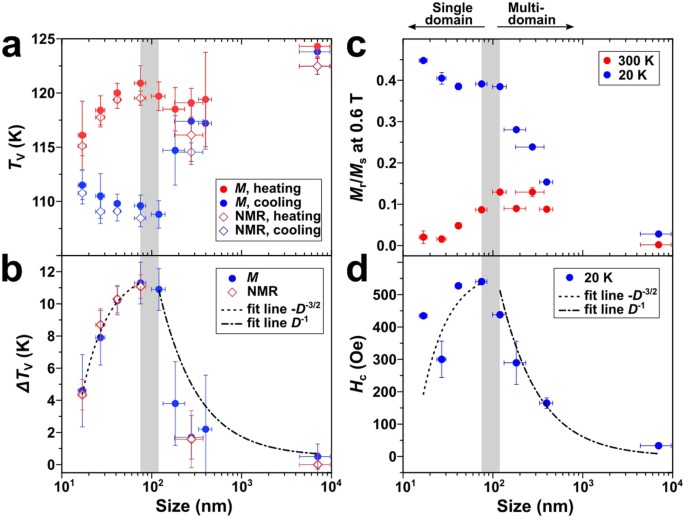
Verway Transition Lead To Semiconducitng To Metallic Behaviour Near 120 K
The largest group of elements is the transition metals. Here is a look at the location of these elements and their shared properties.
For instance, the oldest-known natural magnet, magnetite, demonstrates a fascinating ‘metal-insulator’-type transition near 120–125 K 20, named later as the ‘Verwey transition’ after its. 13 Tu 4/18 EMD pg. 110-120; 13 Th 4/20 M. “Non-volatile ferroelastic switching of the Verwey transition and resistivity of epitaxial Fe3O4/PMN-PT (011)” Scientific Reports 3 (2013) 14 Tu 4/25 Reading Discussion 14 Th 4/27 No reading list. 15 Tu 5/2 No reading list. Two-dimensional (2D) semiconducting transition metal dichalcogenides (TMDCs) and black phosphorus (BP) have beneficial electronic, optical, and physical properties at the few-layer limit.
What Is a Transition Metal?
Of all the groups of elements, the transition metals can be the most confusing to identify because there are different definitions of which elements should be included. According to the IUPAC, a transition metal is any element with a partially filled d electron sub-shell. This describes groups 3 through 12 on the periodic table, although the f-block elements (lanthanides and actinides, below the main body of the periodic table) are also transition metals. The d-block elements are called transition metals, while the lanthanides and actinides are called 'inner transition metals'.
The elements are called 'transition' metals because the English chemistry Charles Bury used the term in 1921 to describe the transition series of elements, which referred to the transition from an inner electron layer with a stable group of 8 electrons to one with 18 electrons or the transition from 18 electrons to 32.
Location of the Transition Metals on the Periodic Table
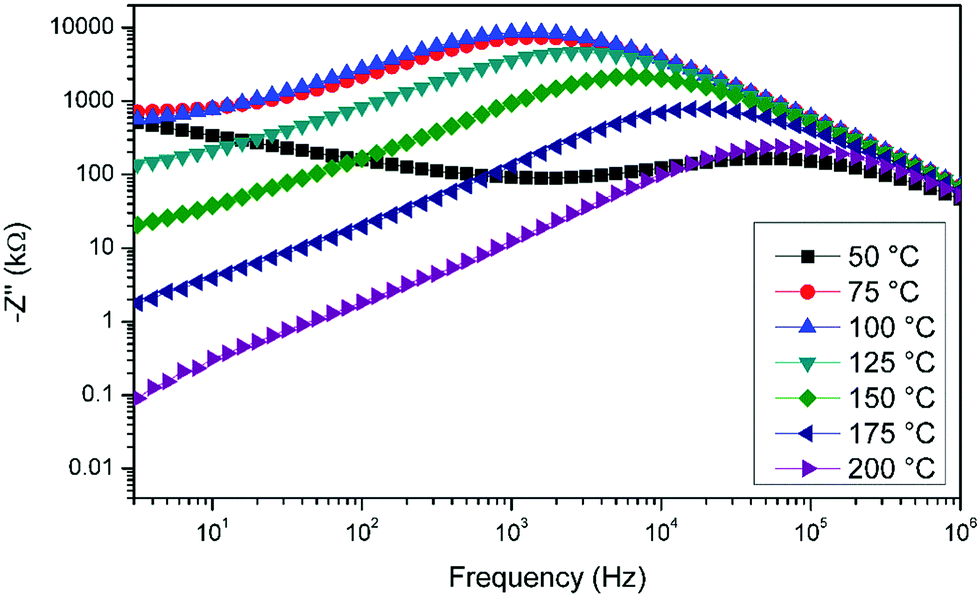
Verwey Transition Lead To Semiconducitng To Metallic Behaviour Near 120 K
The transition elements are located in groups IB to VIIIB of the periodic table. In other words, the transition metals are elements:
- 21 (scandium) through 29 (copper)
- 39 (yttrium) through 47 (silver)
- 57 (lanthanum) through 79 (gold)
- 89 (actinium) through 112 (copernicium) - which includes the lanthanides and actinides
Another way to view it is that the transition metals include the d-block elements, plus many people consider the f-block elements to be a special subset of transition metals. While aluminum, gallium, indium, tin, thallium, lead, bismuth, nihonium, flerovium, moscovium, and livermorium are metals, these 'basic metals' have less metallic character than other metals on the periodic table and tend not to be considered as transition metals.
Overview of Transition Metal Properties
Because they possess the properties of metals, the transition elements are also known as the transition metals. These elements are very hard, with high melting points and boiling points. Moving from left to right across the periodic table, the five d orbitals become more filled. The d electrons are loosely bound, which contributes to the high electrical conductivity and malleability of the transition elements. The transition elements have low ionization energies. They exhibit a wide range of oxidation states or positively charged forms. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. Thus, the complexes form characteristic colored solutions and compounds. Complexation reactions sometimes enhance the relatively low solubility of some compounds.
Quick Summary of the Transition Metal Properties
- Low ionization energies
- Positive oxidation states
- Multiple oxidation states, since there is a low energy gap between them
- Very hard
- Exhibit metallic luster
- High melting points
- High boiling points
- High electrical conductivity
- High thermal conductivity
- Malleable
- Form colored compounds, due to d-d electronic transitions
- Five d orbitals become more filled, from left to right on periodic table
- Typically form paramagnetic compounds because of the unpaired d electrons
- Typically exhibit high catalytic activity